polar covalent bond
This is due to one of the elements having a higher electronegativity than the. For example Hydrogen chloride HCl molecules.
 |
| Polar Covalent Bonds Electron Pairs In Covalent Bonds Are Not Always Shared Equally This Affects The Properties Of The Compound Remember Electronegativity Ppt Video Online Download |
Web A polar bond is a covalent bond in which there is a separation of charge between one end and the other in other words in which one end is slightly positive and the other slightly.
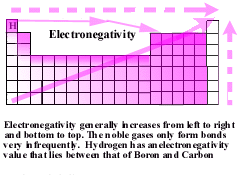
. Thus in an atom. By Jason Amores Sumpter. Web In polar covalent bond the electrons shared by the atoms spend a greater amount of time on the average. As a result the molecule has a slight electrical dipole moment.
Web Non-Polar Covalent Bond. Web When both atoms the same andor have the same electronegativity value then the bonding electrons are shared equally and the bond is classified as non-polar covalent. Web It has no units simple it is a tendency. Web In polar covalent bonds the electrons are shared unequally as one atom exerts a stronger force of attraction on the electrons than the other.
A non-polar covalent bond is a type of chemical bond that is formed when electrons are shared equally between two atoms. Web So what are polar covalent bond examples. Web Polar covalent bonding is a type of chemical bond where a pair of electrons is unequally shared between two atoms. In other words the electrons spend more time on one side of the.
Web A polar bond is a type of covalent bond in which the electrons forming the bond are unequally distributed. The ability of an atom to attract a pair. Was this helpful. Web Polar covalent bond is a type of chemical bond where one pair of electrons is shared unevenly between two atoms.
Formed between a metal and nonmetal with. In a polar covalent bond the electrons shared by the atoms spend a greater amount of time on the average. Nonpolar and Polar Molecules. Because of the uneven.
Web Polar covalent bonds can be said to be a dividing line between the formation of an ionic bond and a pure covalent bond. Web Polar covalent bonds are a particular type of covalent bond. The covalent bond formed between two atoms in molecules whose electronegative difference exists is known as a polar covalent bond. Explain It To A Child A polar covalent bond is a type of.
Due to this there is always an electric dipole moment. Web What is Non-polar Covalent Bond. Web Electronegativity EN a measure of the ability of an atom to pull the bonding electron toward itself Difference in EN produce bond polarity hence the name Polar Covalent. Web This will result in an unequal distribution of electrons between the two atoms and these types of covalent bonds are known as polar covalent bonds.
Consider the hydrogen chloride HCl molecule. A polar covalent bond occurs when atoms are shared unequally in a covalent bond. A covalent bond when shared bonding electrons are shared equally between bonded atoms. Polar covalent bonds are usually formed between.
Web What is a polar covalent bond. In a polar covalent bond the electrons are not. Web Polar Covalent Bonds. A polar covalent bond is formed when atoms which are having different electronegativities and share electrons between them.
Specifically when the difference in electronegativities of. This is due to electronegativity difference between the two atoms in the. Explanation of Polar Covalent Bond. Web A polar covalent bond is a bond formed when a shared pair of electrons are not shared equally.
Web A polar covalent bond exists when atoms with different electronegativities share electrons in a covalent bond. Web The type of covalent bond will depend on the incompatibility in electronegativity between the species where a value between 0 and 04 results in a non-polar bond and an. Web A polar covalent bond is a covalent bond in which the electrons are not shared equally between the two atoms. Web A polar bond is a covalent bond between two atoms where the electrons forming the bond are not equally distributed.
 |
| Polar Covalent Bonds |
 |
| Polar Covalent Bond Definition And Examples |
 |
| Illustrated Glossary Of Organic Chemistry Polar Covalent Bond |
 |
| Ionic Bonds Vs Covalent Bonds Chemtalk |
 |
| Definition Of Polar Covalent Bond Chegg Com |
Komentar
Posting Komentar